878
Views & Citations10
Likes & Shares
What constitutes the code whereby the brain
stores and recalls emotive memory? We suggest that neural recall is based on
the “tripartite” interactions of three physiologic compartments:
· Neurons
- networked, sparse ensembles.
· Neural
Extracellular Matrix (nECM) - A hydrogel with a polysaccharide
lattice surrounding neurons.
· Dopants
(metals and neurotransmitters (NTs)) - ejected from vesicles. The nECM performs
as a “memory material” wherein a neuron imprints incoming cognitive information
as a c dopant code comprising trace metals and NTs (which elicit physiologic as
well as emotive effects).
To “write”, the neuron ejects vesicles
containing dopants into the surrounding nECM, a process analogous to ink-jet
printing on paper. A pattern of metal-centered complexes is “written” within
the nECM around the neurons, effectively encoding cognitive unit(s) of
information (cuinfo).
To “read”, the neuron employs at least 3 types
of “sensors”, aggregates of proteins (i.e., mosaics embedded within its
membrane, examples being GPCR mosaics, K2P channels and acetylcholine receptors
(AcCholR)), all which number many thousands per neuron. They perform as dynamic
chemo-sensors (reported diffusion: 10-1 to 10-3 um2/sec),
which transform the cuinfo code, the “engrams” around individual
neurons, into chemo-electric synaptic signals.
The neural net transforms and consolidates the
chemical signals into coherent psychic states, also instigating reactions of
glands and muscles.
Keywords: Cognitive information, Emotions,
Neurotransmitter, Trace metal, Psycho-chemistry
BACKGROUND
“It was as if I discovered a whole new
universe of chemical elements and had begun to see certain relations between
them, but had no means to organize the whole series into a harmonious and
coherent union.”
-Thomas Wolfe: The Story of a Novel (1936)
“The past is beautiful because one never
realises an emotion at the time. It expands later and thus we do not have
complete emotions about the present, only about the past.”
-Virginia Woolf (1882-1904)
Man may control what he thinks (sometimes),
but not how. How does the brain in animals and man, composed entirely of
matter, experience the psychic talent of persistent recall, to guide behavior?
One could say:
“Mind and memory are inseparable aspects of
the mentating brain.” Leaving aside spirits, ghosts, quantum entanglements and
ethereal entities (literature too vast to cite here), one queries: What happens
between “sensation” and “action”, that we recall as “memory”?
“All mental processes are biological.
Therefore, any disorder must also have a biological basis”.
-Kandel [1]
To the above quote, one could easily
substitute the word “chemical” for “biological”. We modern neuroscientists desire
a unifying principle for memory, but do not want explanations based on ghosts, spirits,
black holes, strings or quantum uncertainty.
Just as today’s medical diagnoses and
clinical treatments are based on chemical considerations, we turn to the
discipline of chemistry for mechanistic clarification of a mental state, such
as memory. After all, who can deny that we are chemical creatures, imbued with
moods and psychic talents that emerge from the chemical reactions in our
brains? The mood-altering drug industry certainly ascribes to this, witness the
multi-billion $ prescription drug market for Ritalin, Prozac, sedatives and the
like, not to mention illegal mood-altering molecules. Curiosity about the
process that generates neural memory has instigated numerous philosophic and
scientific musings [2-11]. For example, Bergson attempted (~1912) to identify
“images” without representation, to measure the interval between matter and
conscious perception [5]. But he wrote his thesis before the age of codes and
coding instigated by Babbage, Turing, Shannon, et al.
And what about emotions? DNA can be
considered as a carrier of “genetic memory” but cannot encode emotions. Marr, a
more contemporary scientist considered “orthogonalizing a set of key vectors”
into higher dimensional space, with “codons” as basic cognitive units (6-8).
The use of the term “codon” was unfortunate in that it suggested that memory
was encoded as a DNA-type information sequence. Marr’s codons and mathematical
equations are equivalent to binary-type synaptic signals with no emotive
signifiers, totally “demotive”. Without dwelling on this overly, it is clear
that a codon cannot encode neural memory, as it does not covey an emotive trace
of past events, of parent to offspring.
Language presents barriers to understanding
the molecular underpinnings of emotions and memory. Explanations become
entangled with the conundrums, inferences and paradoxes of the spoken and
written word (4). Also, words do not apply to non-verbal animals, who also
exhibit “memory”. Rather, we would rather consider a “universal” neural memory
process that is applicable also to non-verbal neural creatures. But what kind
of code can one consider for the “universal” neural net?
We suggest that the issues of neural memory
and emotions be addressed chemo dynamically, with the same terms used to
clarify other biological processes (such as metabolism, photosynthesis, blood
coagulation, reproduction, etc.), cognizant of the kinetics and energetics of
chemical bonds and mechanisms. Notwithstanding, we were inspired to undertake
such a chemical description of mental phenomena, by comments from two
philosophers and one linguist:
• “No shade of emotion is
without bodily reverberations.” - W. James, 1884 [2]
• “Feeling is the basis of
all mental experience.” - S. Langer, 1962 [3]
• “Since the subject is a
physical organism, the system attributed to this must have finite
representation.” – N. Chomsky, 1975 [4]
COMPUTER MODEL
The computer chip’s “memory material” [12-18]
and underlying “information theory” [19-31] establish the concept of a computer
memory code and provided models of physically encoding information as holes,
pits, magnetic orientation, electron spins, phase changes, distribution of
dopants within a matrix, etc. and the design of algorithms to perform (compute)
logical operations. For example, the chip can transform and store electronic
“input” information into physical correlates, encoded by the disposition of the
dopant metals within the chip’s matrix, for recall-on-demand, as electronic
memory. Electrochemical metallization memory chips have been fabricated from
matrices composed of SiO2, WO3, TiO2, etc. Doped with metals (such as Au, Co,
Cu, Ni, PT and W among other) to encode and store information, available for
retrieval. It has been suggested that “memresistive” devices and networks
compute like a brain, suggesting that “they promise to open new directions in
neuromorphic architectures and biological studies “. However, the wired
connections between electronic components that are proposed to store memory are
not analogous to neural synaptic gaps [discussed below in greater detail with
regard to the IBM brain chip and the Blue Brain Project, Marx & Gilon,
2017].
Some assume an
analogy between computer processing and neural mentation [29-31].
“The brain’s
analogue mechanism can be simulated through digital ones” [22].
“Computational
systems are useful … to describe brain processes mathematically” [29].
But something is
lacking in the mathematical treatment of information with its limited encoding
repertoire (0 1). We point out that even at the quantum level, binary formatted
information is monotonic, psychically dead, “flavorless”, “demotive”.
One queries: By
what alchemy could a biochemical process be transmuted into an emotive state?
To date, nobody has
written a mathematical formulation for pain, love, fear, etc., for feelings
that are recalled as emotions. By contrast, logical processes can be affected
through binary-coded algorithms. For example, pain is felt physically with
muscular contractions, pulse changes and an attendant psychic experience, that
are remembered (see conditioning training). But there are no digital codes or
algorithms to simulate an emotive state experienced by a neural creature
experiencing pain, from worm, to snail, to man [1,32]. Though forcefully
suggested by Marr [6-8], emotive states experienced by all neural creatures
appear to be beyond the ken of binary coding. One could say:
“There is no room
between 0 and 1 for emotions”.
ENCODING/DECODING COGNITIVE INFORMATION (COG-INFO): MOLECULAR RECOGNITION
Enter the chemist/physiologist with a palette of signalling molecules (i.e., metal atoms and neurotransmitters (NTs)) that can elicit emotive states. Modern biologists accept that signalling processes are based on molecular recognition, i.e., binding events [33-40]. Expanding on Darwin [41,42], we expect that the complex neural signals that result in emotive mentation and memory, evolved from the signalling processes expressed by more primitive cells. For example, colonies of bacteria employ a number of “modulators”, small molecules that function as signals to instigate group aggregation and tropic responses; for which they also express cognate receptors on their surfaces, effectively “sensors”. A bacterial colony can be considered as a chemo-dynamic aggregate of individual entities that exchange information to maintain contact and coordinate group responses to the environment, by means of chemical signals (Table 1) and cognate sensors.
BACTERIAL PRECURSOR
OF THE NEURAL NET
Continuing in this vein, the brain cab is
considered as an assembly of neurons, whose performance must be governed by the
laws of chemistry and described by the rules of biology. Its conscious state
(of awareness) operates under metabolic conditions and principles similar to
those of an aggregate of bacteria. In that the latter evolved from the former,
it is worth considering the signalling features of the bacterial aggregate and
see how they apply to the neural net.
A bacterial colony feels and responds to its
environment by signalling with molecules (bio modulators) that signal and
instigate group responses (i.e., feelings) to stimuli (Table 1). Here, the discipline of chemistry with its techniques
and theories helps establish biologic facts.
Without delving into the process of cellular
evolution but accepting it as an established Darwinian fact, one could consider
that neural nets, which evolved from bacteria, employ similar chemo-dynamic
signalling modalities. Indeed, analysis revealed that neurons signal one
another with the same biomodulators (now called neurotransmitters (NTs))
employed by bacteria (Table 1) along
with many additional signalling molecules (neuropeptides) (Table 2). Here too, the discipline of chemistry helps establish
the facts of neurobiology.
A clue to the mechanism of neural memory
might reveal an underlying principle applicable to other mental states. Our
“leap” of comprehension (of the psychic states achieved by neural nets), is
based on the neural morphology (extended, arborized shape), that permits many connections
not only with other neuron, but intimate contacts with the surrounding neural
extracellular matrix (nECM), which performs as an archival “memory material”.
An aggregate of bacteria is “conscious”, in
that it “feels” environment and responds by chemical signalling. But the
bacterial aggregate cannot be considered to be “thoughtful”; it has no memory
and cannot recall past stimuli. It responds only to current stimuli with
signalling molecules (Table 1),
sensorially attuned to its environment, conscious in the existential “now”. But
memory requires sets of neurons to recall details of past stimuli. An
increasingly complex memory talent could only emerge from ever more complex
neural structures and signalling (coding) processes. It is not farfetched to
suggest that the evolved neural creatures conserved the core mechanisms of
bacterial signalling [42-49] and developed new ones (Table 2), to perform feats of psychometric signalling, mentation
and memory. For example, C. Elegans,
a primitive organism with 302 neurons has been shown to exhibit memory, the
recall of past conditioning experiences (i.e. tapping, electric shock, [32]).
Presumably, elegans neurons are encased in their own unique nECM, though
characterization has not been reported. It has been established that slime
molds are surrounded by a slime of polanionic polysaccharides, through which
group signalling occurs [50].
Aplysia, a snail with ~20,000 neurons have a
memory, can remember past stimuli and act accordingly [1]. The evolving neural
systems of more complex animals, with ever more neurons organized into sparse
units and specialized anatomic compartments, developed neuropeptides as
additional molecular signals pertaining to the evocation of emotions (complex
psychic states) (Table 2).
Concomitantly, cognate receptors developed on the surfaces to detect the
nECM-tethered NTs, to be discussed further along our narrative. Characterization
of the nECM unique to Aplysia also has not been reported.
The fact that bacterial modulators also serve
as modulators of neural signals, emphasizes that neural mentation processes are
phyto-chemically related to those of bacterial signalling [44-49]. The
modulators (now called NTs) can elicit simultaneous responses from different
cells throughout the body or even under cell culture conditions (see [42] for
the history of the discovery of NTs).Thus, the multi-tasking NT “signal” to
which a neuron responds is entangled with varied responses of other body cells
to the same signal. For example, a list of cell types that respond to ACh would
include neurons, as well as heart, liver, kidney, pulmonary and endothelial
cells. In a neural creature, they all respond to an administered dose of NT not
to be overlooked are the psychic states elicited by the NTs.
NEURONS AND ASTROCYTES (GLIA CELLS)
Though many detailed studies have been
performed to characterize astrocytes, neurons and the nECM (cited here and in
our previous works), none has clarified the phenomenon of central interest: How
is the neural code, which implements mentation and memory, rendered psychically
operative by the interaction of neural cells with their surroundings.
The neurons connect to form a signalling
network employing both synaptic and non-synaptic (ephaptic or “volume
transmission”) signalling modalities [51-58]. The brain’s mental functions are
aided by the “housekeeping” performances of astrocytes/glia cells that
outnumber the neurons 10-fold [59-67]. The astrocytes have been described as
being involved in non-synaptic contacts between neurons. Neuron and glia
interactions regulate neuronal biosynthesis of the nECM and transmission
through it. Thus, glia cells impact short-term and long-term synaptic
connectivity, also correlated to learning and memory.
All these neural cells retain the core chemo
dynamic signalling molecules and cognate receptors of bacteria and help the
neuron to form contiguous networks coupled to sensors or muscles, as
illustrated in Figure 1.
DEFINITIONS
The nECM can be likened to a 3-D lace of organic polymers composed of sulphated glucoseaminoglycans
(GAGs) with foci at the metal-binding centres, who’s dielectric and epitopic aspects is set by the
biosynthesis of those sites. It is noteworthy that the process of metal
complexation occurs in a nanosecond (10-9 s) timeframe.
The term “extracellular space” is misleading,
as it implies an empty vacuum around the neurons. Not only are the neurons
continuously bathed in a watery (serum, lymph) fluid, they are constrained in a
hydrogel lattice comprising a web of glycosaminoglycans (GAGs) in the nECM [68-85]
that permits the binding of cationic metals to encode cog-info, as discussed
below. Thus, the nECM can be likened to a substrate that has been
biosynthesized and treated (i.e., sulphated) so as to prepare metal-binding
sites, which serve as nucleating centres for encoding cognitive information
involving the binding of NTs [85].
By the term “neuron”, we include combinations
of neurons and glia cells that operate in concert to biosynthesize nECM and maintain
the neural synaptic and non-synaptic signalling contacts through the nECM,
whose performance as a chemo-dynamic “memory material” is manifest as
“plasticity”. But the term “synaptic plasticity” (SP) [52, 63, 70] does not
serve as a mechanistic explanation of atomic-scale events. Rather, the
morphologic changes that are observed in neural dendrites serve to augment the
ability of the neuron to interact with the nECM, to recall the code embodied
therein. In rats, SP has been observed in a period 5-10 h after the learning
experience [11]. But perception occurs in a much shorter time frame (i.e., <1
s). Thus, one must look for faster processes for coding/decoding memory, as
discussed below.
The term “imprinting” has been used to
describe the recall of young animals to specific stimuli [11]. But unlike the
classical meaning of the word “printing” (the transfer of ink to paper), the
“imprinting of behavior” is not meant literally but metaphorically i.e., as a
learning process presumed to operate on the basis of repeated synaptic
connectivity (i.e. SP). But this does not provide a mechanistic understanding
of the process of neural recall.
TRIPARTITE MECHANISM
OF MEMORY
Consider a tripartite mechanism [86-93],
whereby the “neuron” marshals the components available to it. These include the
extracellular matrix (nECM in whose lattice it is wrapped) (see above) and the
dopants (such as metals and NTs), which the neuron accumulates within vesicles,
which it ejects. With these, the neuron encodes molecular (rather than
cellular) building blocks memory.
A chemographic notation, which describes the
chemical basis of the neural memory code as cuinfo, is presented (Figure 2).
The nECM can be likened to a 3-D lace of
organic polymers composed of sulfated glucoseaminoglycans (GAGs) that serve as
metal-binding centers. In emotive memory, NTs that complex with the metals
within the cuinfo, are released from vesicles and are
available to form cuinfo:NT complexes. It is noteworthy that the
chemical processes of metal complexation occur in a nanosecond (10-7)
timeframe. Thus, it is much faster than neural signalling and would not impede
neural communications.
“WRITING” NEURAL
MEMORY
We propose that the “writing” of cuinfo occurs
by neural ejection of vesicles.
The presynaptic neuron (the one that gets an
action potential signal) “writes” cuinfo by ejecting the content of
vesicles [94-118] which contain metals and NTs, to specific addresses within
the nECM (Figure 3A and B). The only
known function of synaptic vesicles is to release neurotransmitters and metals
into the nECM [101,102]. The metals are released into the nECM GAGs that have
specific pattern of varied planar orientations or densities corresponding to
the location of the sulphate groups. Like “inkjet printing” which is based on
the piezoelectric dispersion of colored inks as droplets deposited onto paper (Figure 3C).
Other workers have suggested that chemical
modulators are involved in imprinting memory [113-120], but details of this
process need to be clarified. We use the term “neuron” to include the
astrocytes (glial cells) that have been shown to release “gliatransmitters”,
glutamate and ATP [117].
“READING” NEURAL
MEMORY
It has been pointed out that cell membranes
act as signalling platforms. In that vein, we propose that “reading” of cuinfo
occurs by virtue of the many sensors embedded within the neural membrane. They
detect and decode the various metal-centered complexes contained within the
nECM. Based on the literature, we have identified 3 classes of chemo dynamic
sensors embedded within the neural membrane, as discussed below:
1. GPCR receptors [121-150]: These are
multimeric proteins (dimers, tetramers, mosaics, aggregates) whose canonical
motifs are based on 3 domains: An extracellular domain, extending more than 50Å
into the nECM; a transmembrane domain (i.e. 7-helical barrels penetrating the
membrane; an intracellular domain, coupled to ATP/GTP metabolism, capable of
signalling to its own nucleus as well as to other neurons.
2. The NTs are the molecular equivalents of
emotive states, which the GPCRs can detect. The external facet of the moving
GPCR sensor (Figure 4) is sensitive
to pattern of NTs tethered to the cuinfo in the nECM. More than 800
distinct types of GPCRs have been identified; neurons express millions of these
on their surface (Table 3). The
seven-transmembrane helix structure of the primitive bacterio-rhodopsin sensor
motif is conserved and adapted in all GPCR types (Figure 5A).
Assuming a 10x10Å size of a cuinfo,
this would translate into a “reading” of 105 to 107 cuinfo/sec
by a single mosaic. Considering that the neuron expresses many thousands of
mosaics on its surface, the numbers suggest that the neuron can effectively
refresh its recall of many stored memory units.
As the GPCR aggregates are associated with
ion channels, they can transduce chemical affinities for tethered ligands (like
affinity chromatography [121], into mini-gating responses related to
mini-electric action potentials relating to short-term memory. Some are
functionally connected to the cell nucleus, to instigate the biosynthesis of
new nECM and proteins, the basis of persistent, long term memory. The GPCRs can
perform like dynamic switches, combining and recombining into circuits,
diffusing within and through the lipid bilayer surface of the neuron, in continuous
contact with the surrounding nECM, “perusing” the exposed cuinfo as they
traverse along the exposed nECM.
K+
Channels [151-158]:
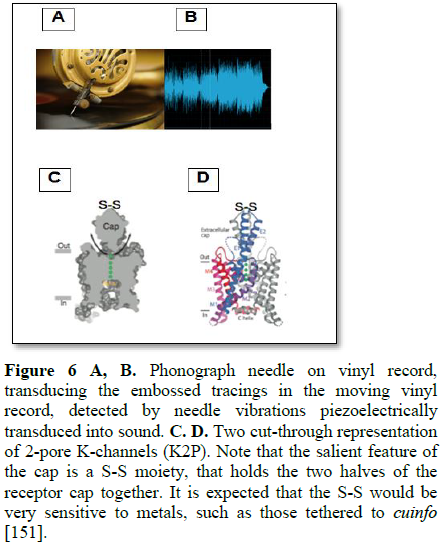
Consider a phonograph needle capable of
sensing engraved tracings in a vinyl record with a sharp needle, to transduce
tracings into sound (Figure 6A and B).
The 2-pore K-channels (K2P) exhibit a
structure expected of a sensor. They are organized as 3-domains; an
extracellular domain (a sensor cap with an S-S “needle”), a transmembrane
region and an intracellular domain. The cap structure extends 35 Å into the extracellular domain and
exposes the S-S moiety at its tip to the metals tethered to the cuinfo within
the nECM.
structure extends 35 Å into the extracellular domain and exposes the S-S moiety at
its tip to the metals tethered to the cuinfo within the nECM.
The reactivity of the S-S moiety to metals is
well known to chemists. Allosteric flexing induced by the “S-S tip” as it
adsorbs/desorbs to a tethered metal cation (of a cuinfo) during its
traverse of the membrane, could transduce into a mini-gating event, affecting
electrical signalling to the neural net, as for example, the tiny spikes around
the major action potential spikes. The S-S bond is relatively weak compared to
a C-C bond and could be sensitive to different metals entrapped by the cuinfo,
as exemplified by the reactions of S-S moieties with soluble metals or metal
surfaces (Figure 4).
Recent x-ray crystallography findings
confirmed this model. The K2P channel was shown to be modulated by a drug
(Prozac) which binds to the junction of the channel, where it merges with the
membrane of the neuron [156,157]. Interestingly, the side effects of Prozac are
loss of memory and changes in psychic states. The binding of Prozac to K2P
appears to interfere with the motility of the extracellular region, specifically
with the cuinfo-reading S-S moiety at the tip of the K2P. Loss of this
reading ability is mirrored by forgetting.
Acetylcholine
receptors (AChR) [159-167]
The nAChR neuro-receptor is a well-studied
ligand-gated ion channel that opens upon acetylcholine binding, and is
responsive to the cationic acetylcholine (Figures
7A and B).
The exterior facet of the receptor AChR is
anionic, presents a 10Å wide, negatively charged face to the outside. This
serves to attract the cationic AChol into the channel to become attached to the
ligand-binding site. But the negative facet of the AChR could also respond and
sense positively charged side chains from NTs tethered to a cuinfo through
a metal, as illustrated in Figure 7.
Its affinity for Ca+2 and other
cationic metals, due to its anionic surface and internal channels, make it
capable of sensing and allosterically decoding a cuinfo that expresses a
cationic ammonium group (R3NH+) (i.e. (e.g. secondary
amine in Epi or Arg and Lys neuropeptides) (Figure
8). One might also expect that its affinity for the cationic ACh is
mirrored by a (milder) response to Arg groups presented by NTs tethered to the
nECM. The detected moiety instigates a gated mini-signal to the neural net. The
opening and closing of ligand and voltage gated ion channel proteins causes
small electrical potential (resistivity) changes on a membrane resulting in
mini-electrical signals.
The exterior facet of the receptor AChR is
anionic, presents a 10Å wide, negatively charged face to the outside. This
serves to attract the cationic AChol into the channel to become attached to the
ligand-binding site. But the negative facet of the AChR could also respond to
select side chains from NTs tethered to a cuinfo through a metal.
SUMMARY OF
RECEPTORS/SENSORS
We describe 3 types of membrane-embedded
organelles that are involved in chemo dynamic neural “perusing” of the
cog-info encoded within the nECM around the
neuron. There may be other types “perusing” modes.
As regards the history of these receptors, the bacterial system has a number of receptors located within their surface membranes, as well as ion channels, all which evolved along with the neuron to form the signal sensing organelles (Table 3). Thus, feelings are experienced by neurons via the signalling properties of bio modulators (i.e. NTs) interacting with cognate receptors (Table 3), a system that evolved from bacteria (35-42), but adapted and added to, by the neurons to encode, evoke and remember emotive memory.
DISCUSSION
Memory can be classified as a psychic
experience instigated by the senses, which is recalled. Some presume that
memory is stored in the neuron; others opine that memory is stored as an
activity of a neural circuit, though such a “memory circuit” has not been
realized for electronic circuits. Of course, synaptic plasticity (SP) must be
involved in the various stages of the processing of cognitive information by
the neuron. However, the terms SP and its variant “long term potentiation (LTP)
do not describe the molecular features by which cog-info is encoded. Rather, it
describes the increased ability of the neuron to interact with its
surroundings, to decode the cog-info embodied in the nECM and to signal
neighboring neurons (see: synaptic contact).
Dogmas of
Neurobiology
In keeping with the modern approach to
medicine and clinical practice, one cannot simply overlook the explanatory role
of biochemistry in elucidating mental processes.
Q: What are the doctrinal guidelines that
a chemist refers to when advocating a possible mechanism regarding psychic
neural processes?
In particular, the dogmas of neurobiology
must be questioned, particularly:
• Cajal’s model of neural
signaling exclusively through synaptic contacts.
• Synaptic plasticity-a la
Hebb and Kandel
Q: How can one describe the molecular
features of brain that function to generate memory?
A: Establish facts – identify critical
components/parameters. Then weave the facts into a concept of operation that
conforms to the possible chemical interactions available to the neuron. Much
has been said about the electrodynamic signalling between synaptically
connected neurons. But this is incomplete description. Chemodynamic
interactions of the neurons with the nECM, as described by a chemical
recognition theory [33-40].
Q: How does one account for emotions,
which have no coding option in the binary world; how are emotions embodied and
encoded in the neural system?
A: Neurotransmitters (NTs) (also called
“biomodulators)”, are the only molecules in the neuron’s repertoire which can
affect physiologic reactions and elicit psychic states/emotional responses.
With the exception of acetylcholine which is cationic, most bio modulators
contain electron-rich ligands, avid for cationic metals, either free or
tethered to a matrix (as in affinity chromatography).
Four criteria for characterizing NTs
1. Biosynthesized
in or accumulated by the neuron, stored in vesicles.
2. Released from
the vesicles in sufficient quantities to produce a significant (measurable)
effect on the postsynaptic cell.
3. Artificial
administration of NTs mimics natural release (elicit physiologic and emotive
responses).
4. A mechanism
exists for NT removal from the synaptic cleft.
Q: What are we,
that we can recapitulate our life experiences through memory?
A: We are a
collection of cells composed of molecules and atoms interacting in a particular
way to generate, store and recall psychic experiences, remembered to achieve
survival.
The activated
neurons “write” by releasing dopant-loaded vesicles into the nECM. The vesicles,
which traverse the membrane, are loaded with trace metals and NTs; the neuron
controls the location and level of encoders released into the nECM, reminiscent
of ink-jet printing of different colors by focused piezo-electric impulses.
Neurons chemo
dynamically “read” the nECM via sensor aggregates that move laterally within
the membrane lipid bilayer. The nECM structures may be viewed as molecular
scaffolds whereby varied planar orientations or densities of the sulphate
groups can achieve metal binding interactions which in turn affect affinities
for various ligands [85]. Significant inroads have been made in the sequencing
of GAGs and encoded sequences. The external facets of the sensors contact
facets of the nECM. As they diffuse over the membrane, they allosterically
“recognize” the cuinfo at each particular “address” in the nECM; the
chemically-induced resonance states of individual neurons are communicated to
neural network via electrodynamic signalling pathways.
One could consider the process in musical terms. The nECM is the “partitura”, the score which the neuron reads with its many dynamic sensors (Table 3), like a multi-stringed instrument (Figure 9), each string capable of generating a unique tone but resonating with others to generate harmonic overtones. And like music, the “note” must be considered in the context of a set, whose pattern is ‘read’ by the neuron to generate the experience of memory.
The sensors (receptors) perform as biologic
“switches which can combine into aggregates (“circuits”) to mentate the
individual neuron’s response to chemical signals decoded from the nECM. Some of
the sensors are capable of decoding the emotive quality of memory by virtue of
their affinity for tethered NTs; others may respond only to metals. Multiples
of such aggregates, which are associated with ion-channel gates, traverse the
neural surface to “read” the nECM. They effectively process the “chemical
algorithms” whereby the neural circuit mentates.
CONCLUSION
Is the search for a universal mechanism of
neural memory misguided? Are we forbidden by fear of Descarte’s Mind/Body
conundrum? Based on everything we know about the chemical basis of all
biological processes, the metaphor of an electronic artefact programmed in
binary code is inadequate to describe neural mental activities, as it lacks
emotive qualities (see current discussion of this in Science, April 2018 (174)).
We meld the observations of
neuro-morphologists (particularly Triller et al. [124-126] and Vizi [132,133])
with the concepts of the chemist, to present a coherent mechanism that
describes how cog-info can be encoded (written), stored and decoded (read). To
that end, we envision 4 tasks:
·
Define “emotions” with a molecular
vocabulary.
·
Identify a neural “memory material”
wherein persistent memory is stored.
·
Describe a neural encoding (writing)
process.
·
Describe a neural decoding (reading)
process.
The tripartite mechanism copes with these
points by positing that the neuron forms metal-centered complexes (cuinfo)
within the nECM around itself. Expectedly, each metal instigates a unique
binding structure with each of the many (>100) NTs and to the nECM. There is much evidence that NTs can elicit
physiologic responses as well as emotive states. Thus, it is not farfetched to
suggest that each cuinfo: NT presents a unique “ligand pattern” with
emotive context, which is sensed by the neural surface (sensors) to reconstruct
past experience as memory.
The psychic states achieved by neurons are stored as memory, signalled to the neural net, as schematically presented in Figure 10.
The heuristic implications of such a complex,
chemo-electric signalling process are manifold. For example, a demonstration of
an electrodynamic effect modulated by the interactions of metals with NTs or
polysaccharides (as models of the nECM) would augment the credibility of the
tripartite mechanism of neural memory. Such work is underway with our collaborators
as per the initial reports [168].
ACKNOWLEGEMENT
(By GM). A memorium to my wife and fan, the
artist Georgette Batlle (1940-2009), whose drawings helped me visualize
interactions of blood clotting factors modulated by metals, instigating an
epiphany regarding psycho-chemical processes. Thanks to friends, Lilly Rivlin
(New York, N.Y.) and the late Bill Needle (Eastchester, N.Y.) for their early
encouragement and financial support in the period 1980-1984. I appreciate
ongoing discussions with Karine Ahouva Leopold (Paris), regarding the
distinction between feelings, emotions and behaviour.
CONFLICT OF INTEREST
GM is a founder of MX Biotech Ltd., with the
commercial goal to develop new classes of “memory materials” and devices.
CG is emeritus professor of HU, but is active
in developing and patenting peptide-based tools for surgery and pharmacology.
Notwithstanding, the ideas forwarded here are
scientifically genuine and presented in good faith, without commercial clouding
of the concepts expressed herein.
1.
Kandel ER (2006) In Search of Memory. W.W.
Norton & Co., New York.
2.
James W (1884) What is an emotion? MIND 9:
188-206.
3.
Langer SK (1962) Philosophical Sketches.
Johns Hopkins Press, Baltimore, MD.
4.
Chomsky N (1975) Reflections on language.
Pantheon Books, New York.
5.
Bergson H (2004) Matter and memory (translation
from French by Paul NM and Palmer WS 1912). Dover Philosophic Classics.
6.
Marr D (1970) A theory for cerebral
neocortex. Proc R Soc Lond 176: 161-234.
7.
Marr D (1971) Simple memory: A theory for
archicortex. Philos Trans R Soc Lond B Biol Sci 262: 23-81.
8.
Willshaw DJ, Dayan P, Morris RGM (2015)
Memory, modelling and Marr: A commentary on Marr (1971) ‘Simple memory: A
theory for archicortex’. Philos Trans R Soc Lond B Biol Sci 370: 20140383.
9.
Dehaene S (2014) Consciousness and the
brain. Deciphering how the brain codes our thoughts. Penguin Books, New York.
10. Rosenblum
B, Kuttner F (2011) Quantum enigma: Physics encounters consciousness. Oxford
University Press, UK.
11. Horn G
(2004) Pathways of the past: The imprinting of memory. Nature Rev Neuroscience
5: 108-121.
12. Ventra
MD, Pershin YY (2011) Memory materials: A unifying description. Mater Today 14:
584-591.
13. Valov
I, Waser R, Jameson JR, Kozicki MN (2011) Electrochemical metallization
memories - Fundamentals, applications, prospects. Nanotechnology 22: 254003.
14. Di
Ventra M, Pershin YV (2013) Memcomputing: A computing paradigm to store and
process information on the same physical platform: 1211.
15. Valov
I, Kozicki MN (2013) Cation-based resistance change memory. J Phys D Appl Phys
46: 074005.
16. Seo S,
Yoon Y, Lee J, Park Y, Lee H (2013) Nitrogen-doped partially reduced graphene
oxide rewritable nonvolatile memory. ACS Nano 7: 3607-3615.
17. Lin WP,
Liu SJ, Gong T, Zhao Q, Huang W (2014) Polymer-based resistive memory materials
and devices. Adv Mater 26: 570-606.
18. Traversa
FL, Di Ventra M (2014) Universal Memcomputing machines. IEEE Trans Neural Netw
Learn Syst 26: 2702-2715.
19. Boole G
(2005) The laws of thought: The mathematical theories of logic and probabilities
1853. Project Gutenberg.
20. Turing
A (1936) On computable numbers, with an application to the Entscheidungs
problem. Proc Lond Math Soc 42: 230-265.
21. McCulloch,
Pitts WS (1943) A logical calculus of the ideas immanent in nervous activity.
Bull Math Biophys 7: 115-133.
22. Neumann
JV (1958) The computer and the brain. Yale University Press, New Haven CT.
23. Gleick
J (2011) The information. Pantheon Books, New York.
24. Landauer
R (1991) Information is physical. Phys Today 44: 93-29.
25. Landauer
R (1991) The physical nature of information. Phys Lett A 217: 188-193.
26. Picard
R (1997) Affective computing. MIT Press, Boston.
27. Kleine
CC (2006) Recognition and simulation of emotions seminar: Human-robot
interaction. SoSe Fachbereich Informatik Universitat Dortmund.
28. Levy S (1992) Artificial life. Random house,
New York.
29. Guidolin
D, Albertin G, Guescini M, Fuxe K, Agnati LF (2011) Central nervous system and
computation. Q Rev Biol 86: 265-285.
30. Pockett
S (2014) Problems with theories that equate consciousness with information or
information processing. Front Syst Neurosci 8: 225.
31. Carleo
G, Troyer M (2017) Solving the quantum many-body problem with artificial neural
networks. Science 355: 602-606.
32. Ardiel
EL, Rankin CH (2010) An elegant mind: Learning and memory in Caenorhabditiselegans.
Learn Mem 17: 191-201.
33. Katchalski
E (1992) Molecular surface recognition: Determination of geometric fit between
proteins and their ligands by correlation techniques. Proc Natl Acad Sci USA
89: 2195-2199.
34. Juliano
RI, Haskill S (1993) Signal transduction from extracellular matrix. J Cell Biol
120: 577-585.
35. Lobmaier
C, Hawa G, Götzinger M, Wirth M, Pittner F, et al. (2001) Direct monitoring of
molecular recognition processes using fluorescence enhancement at
colloid-coated microplates. J Mol Recognit 14: 215-222.
36. Gooding
JJ, Hibbert DB, Yang W (2001) Electrochemical metal ion sensors. Exploiting
amino acids and peptides as recognition elements. Sensors 1.
37. VonKorff
M, Steger M (2004) Pharmacophore pattern recognition of small molecular
ligands. J Chem Inf Comput Sci 44: 1137-1147.
38. Ma Z,
Jacobsen FE, Giedroc DP (2009) Coordination chemistry of bacterial metal
transport and sensing. Chem Rev 109: 4644-4681.
39. Marcos
V, Stephens AJ, Jaramillo-Garcia J1, Nussbaumer AL, Woltering SL, et al. (2016)
Allosteric initiation and regulation of catalysis with a molecular knot.
Science 352: 1555-1559.
40. Aschner M (2008) The functional significance
of brain metallothioneins. FASEB J 10: 1129-1136.
41. Romanes
GJ (1883) Mental evolution in animals with posthumous essay on instinct by
Charles Darwin. Kegan, Paul, Trench & Co., London. Nabu Public Domain
Reprints.
42. Zakon
HH (2012) Adaptive evolution of voltage-gated sodium channels: The first 800
million years. Proc Natl Acad Sci U S A 109: 10619-10625.
43. Valenstein
ES (2005) The war of the soups and the sparks. The discovery of
neurotransmitters. Columbia University Press, New York.
44. Lefkowitz
RJ (2004) Historical review: A brief history and personal retrospective of
seven-transmembrane receptors. Trends Pharmacol Sci 25: 413-423.
45. Reith
ME (2002) Neurotransmitter transporters: Structure, function and regulation.
Springer-Verlag, New York.
46. Mustafa
AK, Gadalla MM, Snyder H (2009) Signaling by gasotransmitters. Sci Signal 2.
47. Corringer
J, Poitevin F, Prevost M, Sauguet L, Delarue M, et al. (2012) Structure and
pharmacology of pentameric receptor channels: From bacteria to brain. Structure
20: 941-956.
48. Zhang
Z, Wu J, Yu J, Xiao J (2012) A brief review on the evolution of GPCR:
Conservation and diversification. Pharmacol Rev 52: 63-89.
49. Fotiadis
D, Qian P, Philippsen A, Bullough PA, Engel A, et al. (2004) Structural
analysis of the reaction center light-harvesting complex I photosynthetic core
complex of Rhodospirillumrubrum using atomic force microscopy. J Biol Chem 279:
2063-2068.
50. Brown
SP, Blackwell HE, Hammer BK (2018) The State of the Union Is Strong: A review
of ASM's 6th Conference on Cell-Cell Communication in Bacteria. J Bacteriol
200: e00291-e00318.
51. Koch C,
Zador A (1993) The function of dendritic spines: Devices subserving biochemical
rather than electrical compartmentalization. Neuroscience 13: 413-422.
52. Stepanyants
A, Hof PR, Chklovskii DB (2002) Geometry and structural plasticity of synaptic
connectivity. Neuron 34: 275-288.
53. Milo R,
Itzkovitz S, Kashtan N, Chklovskii D, Alon U, et al. (2002) Network motifs:
Simple building blocks of complex networks. Science 298: 824-827.
54. Fuxe K,
Dahlström A, Höistad M, Marcellino D, Jansson A, et al. (2007) From the
Golgi-Cajal mapping to the transmitter-based characterization of the neuronal
networks leading to two modes of brain communication: Wiring and volume
transmission (VT). Brain Res Rev 55: 17-54.
55. Purves
D, Augustine GJ, Fitzpatrick D, Katz LC, LaMantia A, et al. (2001)
Neuroscience. Sinauer Associates, Sunderland (MA).
56. Brady
S, Albers WR, Price D (2011) Basic Neurochemistry: Principles of Molecular,
Cellular and Medical Neurobiology. Elsevier Science, New York.
57. Goyal
RK, Chaudhury A (2013) Structure activity relationship of synaptic and
junctional neurotransmission. Auton Neurosci 176: 11-13.
58. Cajal R
(1995) Histology of the nervous system of man and vertebrae. Oxford University
Press.
59. Giaume
C, Koulakoff A, Roux L, Holcman D, Rouach N (2010) Astroglial networks: A step
further in neuroglial and gliovascular interactions. Nat Rev Neurosci 11:
87-99.
60. Li D,
Agulhon C, Schmidt E, Oheim M, Ropert N (2013) New tools for investigating
astrocyte-to-neuron communication. Front Cell Neurosci 7: 193-210.
61. Vargova
L, Sykova E (2014) Astrocytes and extracellular matrix in extra synaptic volume
transmission. Philos Trans R Soc Lond B Biol Sci 369: 20130608.
62. Hirase
H, Iwai Y, Takata N, Shinohara Y, Mishima T (2014) Volume transmission
signaling via astrocytes. Philos Trans R Soc Lond B Biol Sci 369: 20130604.
63. Haydon
PG, Nedergaard M (2015) How do astrocytes participate in neural plasticity?
Cold Spring Harb Perspect Biol 7: a020438.
64. Martín
R, Bajo-Grañeras R, Moratalla R, Perea G, Araque A (2015) Circuit-specific
signaling in astrocyte-neuron networks in basal ganglia pathways. Science 349:
730-734.
65. Stevens
B, Muthukumar AK (2016) Differences among astrocytes. Science 351: 813.
66. Perea G,
Navarette M, Araque A (2009) Tripartite synapses: Astrocytes process and
control synaptic information. Trends Neurosci 32: 421-431.
67. Halassa
MM, Fellin T, Haydon PG (2009) Tripartite synapses: Roles for strocytes: Roles
for astrocytic purines in control of synaptic physiology and behavior.
Neuropharmacol 57: 343-346.
68. Schmitt
FO (1962) Macromolecular specificity and biological memory. MIT Press,
Cambridge, MA.
69. Bogoch
S (1968) The biochemistry of memory: With an inquiry into the function of brain
mucoids. Oxford University Press, London.
70. Diyatev
A, Schachner M (2003) Extracellular matrix molecules and synaptic plasticity.
Nat Rev Neurosci 4: 456-469.
71. Dityatev
A, Seidenbecher CI, Schachner M (2010) Compartmentalization from the outside:
The extracellular matrix and functional microdomains in the brain. Trends
Neurosci 33: 503-512.
72. Kleene
G, Schachner M (2004) Glycans and neural cell interactions. Nat Rev Neurosci 5:
195-209.
73. Viapiano
MS, Matthews RT (2006) From barriers to bridges: Chondroitin sulfate proteoglycans
in neuropathology. Trends Mol Med 12: 488-496.
74. Deepa
S, Carulli D, Galtrey C, Rhodes K, et al. (2006) Composition of perineuronal
net extracellular matrix in rat brain: A different disaccharide composition for
the net-associated proteoglycans. J Biol Chem 281: 17789-17800.
75. Gogolla
N, Caroni P, Lüthi A, Herry C (2009) Perineuronal nets protect fear memories
from erasure. Science 325: 1258-1261.
76. Avram,
Shaposhnikov S, Buiu, C, Mernea M (2014) Chondroitin sulfate proteoglycans:
Structure-function relationship with implication in neural development and
brain disorders. BioMed Res Int 2014.
77. Theocharis
AD, Skandalis SS, Gialeli C, Karamanos NK (2016) Extracellular matrix
structure. Advanced Drug Delivery Reviews 97: 4-27.
78. Suttkus
A, Morawski M, Arendt T (2016) Protective properties of neural extracellular
matrix. Mol Neurobiol 53: 73-82.
79. Dzyubenko
E, Gottschling C, Faissner A (2016) Neuron-glia interactions in neural
plasticity: Contributions of neural extracellular matrix and perineuronal nets.
Neural Plast 2016.
80. De Luca
C, Papa M (2017) Matrix metalloproteinases, neural extracellular matrix and
central nervous system pathology. Prog Mol Biol Translat Sci 14: 167-202.
81. Kamali
P, Nicholson C (2013) Brain extracellular space: Geometry, matrix and
physiologic importance. Basic Clin Neurosci 4: 282-286.
82. Maroudas
A, Weinberg PD, Parker KH, Winlove CP (1988) The distributions and
diffusivities of small ions in chondroitin sulphate, hyaluronate and some
proteoglycan solutions. Biophys Chem 32: 257-270.
83. Gama
CI, Tully SE, Sotogaku N, Rawat M, et al. (2006) Sulfation patterns of
glycosaminoglycans encode molecular recognition and activity. Nat Chem Biol 2:
467-474.
84. Miyata
S, Nadanaka S, Igarashi M and Kitagawa H (2018) Structural variation of
chondroitin sulfate chains contributes to the molecular heterogeneity of
perineuronal nets. Front Integr Neurosci 12: 3.
85. Zhang
F, Liang X, Beaudet JM, Lee Y, Linhardt RJ (2014) The effects of metal ions on
heparin/heparin sulfate-protein interactions. J Biomed Technol Res 1: 1-15.
86. Marx G,
Gilon C (2012) The molecular basis of memory. ACS Chem Neurosci 3: 633-642.
87. Marx G,
Gilon C (2013) The molecular basis of memory. MBM Part 2: The chemistry of the
tripartite mechanism. ACS Chem Neurosci 4: 983-993.
88. Marx G,
Gilon C (2014) The molecular basis of memory. MBM Part 3: Tagging with
neurotransmitters. Front Aging Neurosci 6: 58.
89. Marx G,
Gilon C (2016) The molecular basis of neural memory. MBM Part 4: The brain is
not a computer. Binary computation versus “multinary” mentation. Neurosci
Biomed Eng 4: 14-24.
90. Marx G,
Gilon C (2016) The molecular basis of neural memory. MBM Part 6: Emotive and
rational modes. Int J Neurology Res 2: 259-268.
91. Marx G,
Gilon C (2017) The molecular basis of neural memory. MBM Part 7: Artificial
intelligence (AI) versus neural intelligence (NI). AIMS Med Sci 4: 254-273.
92. Marx G,
Gilon C (2018) The molecular basis of neural memory. Part 10. The sins and
redemption of Neurobiology. J Neurol Neurocrit Care 1: 1-7.
93. Kandel
ER, Dudai Y, Mayford MR (2014) The molecular and systems biology of memory.
Cell 157: 163-186.
94. Segal M
(2017) Dendritic spines: Morphological building blocks of memory. Neurobiol
Learn Mem 138: 3-9.
95. Cole
TB, Wenzel HJ, Kafer KR, Schwartzkroin PA, Palmiter RD (1999) Elimination of
zinc from synaptic vesicles in the intact mouse brain by disruption of the ZnT3
gene. Proc Natl Acad Sc USA 96: 1716-1721.
96. Matteoli
M, Coco S, Schenk U, Verderio C (2014) Vesicle turnover in developing neurons:
How to build a presynaptic terminal. Trends Cell Biol 14: 133-140.
97. Bruns
D, Jahn R (1995) Real-time measurement of transmitter release from single
synaptic vesicles. Nature 374: 62-65.
98. De-Miguel
FF, Leon-Pinzon C, Noguez P, Mendez B (2015) Serotonin release from the
neuronal cell body and its long-lasting effects on the nervous system. Philos
Trans R Soc Lond B Biol Sci 370.
99. Bruns
D, Riedel D, Klingauf J, Jahn R (2000) Quantal release of serotonin. Neuron 28:
205-220.
100. Lasiecka
ZM, Winckler B (2011) Mechanisms of polarized membrane trafficking in
neurons-Focusing in on endosomes. Mol Cell Neurosci 48: 278-287.
101. Sudhof
TC, Rizo J (2011) Synaptic vesicle exocytosis. Cold Spring Harb Perspect Biol
3: a005637.
102. Südhof
TC (2013) Neurotransmitter release: The last millisecond in the life of a
synaptic vesicle. Neuron 80: 675-690.
103. Trueta
C, De-Miguel FF (2013) Extra synaptic exocytosis and its mechanisms: A source
of molecules mediating volume transmission in the nervous system. Front Physiol
3: 319.
104. Trueta C, De-Miguel FF (2012) Extrasynaptic
exocytosis and its mechanisms: A source of molecules mediating volume
transmission in the nervous system. Front Physiol 3: 319.
105. Wilhelm
BG, Mandad S, Kröhnert K, Schäfer C, Rammner B, et al. (2014) Composition of
isolated synaptic boutons reveals the amounts of vesicle trafficking proteins.
Science 344: 1023-1028.
106. Agnati LF, Fuxe (2014) Extracellular-vesicle
type of volume transmission and tunnelling-nanotube type of wiring transmission
add a new dimension to brain neuro-glial networks. Philos Trans R Soc Lond B
Biol Sci 369: 20130505.
107. Prada
I, Amin L, Furlan R, Legname G, Verderio C (2016) A new approach to follow a
single extracellular vesicle-cell interaction using optical tweezers.
Biotechniques 60: 35-41.
108. Farsi
Z, Preobraschenski J, van den Bogaart G, Riedel D, Jahn R (2016) Woehler A.
Single-vesicle imaging reveals different transport mechanisms between
glutamatergic and ABAergic vesicles. Science 351: 981-984.
109. Pan E,
Zhang XA, Huang Z, Krezel A, Zhao M, et al. (2011) Vesicular zinc promotes
presynaptic and inhibits postsynaptic long-term potentiation of mossy fiber-CA3
synapse. Neuron 71: 1116-1126.
110. Mei Y,
Frederickson CJ, Giblin LJ, John HW, Yuliya M, et al. (2011) Sensitive and
selective detection of zinc ions in neuronal vesicles using PYDPY1, a simple
turn-on dipyrrin. Chem Commun 47: 7107-7109.
111. Kaeser
PS, Regehr WG (2014) Molecular mechanisms for synchronous, asynchronous and
spontaneous neurotransmitter release. Annu Rev Physiol 76: 333-363.
112. Volknandt
W (1995) The synaptic vesicle and its targets. Neuroscience 64: 277-300.
113. Kavalali
ET (2015) The mechanisms and functions of spontaneous neurotransmitter release.
Nat Rev 16: 5-17.
114. Rizo J,
Xu J (2015) The synaptic vesicle release machinery. Annu Rev Biophys 44:
339-367.
115. Davis
GW, Muller M (2015). Homeostatic control of presynaptic neurotransmitter
release. Ann Rev Physiol 77: 251-270.
116. Borroto-Escuela
DO, Agnati LF, Bechter K, Jansson A, Tarakanov A, et al. (2015). The role of
transmitter diffusion and flow versus extracellular vesicles in volume
transmission in the brain neural-glial networks. Philos Trans R Soc Lond B Biol
Sci: 370.
117. Covelo
A, Araque A (2018) Neuronal activity determines distinct gliotransmitter
release from a single astrocyte. Elife 7: e32237.
118. Meredith
RM, McCabe BJ, Kendrick KM, Horn G (2014) Amino acid neurotransmitter release
and learning: A study of visual imprinting. Neuroscience 126: 249-256.
119. Knipp
M, Roschitzki GB, Vašák M, Meloni G (2005) Zn7Metallothionein-3 and the
synaptic vesicle cycle: Interaction of metallothionein-3 with the small GTPase
Rab3A.
120. György
C (2011) The biological basis and clinical significance of hormonal imprinting,
an epigenetic process. Clin Epigenetics 2: 187-196.
121. Caron
MG, Srinivasan Y, Pitha J, Kociolek K, Lekowitz RJ (1979) Affinity
chromatography of the β-adregenic receptor. J Biol Chem 254: 2923-2927.
122. Kroeze
WK, Sheffler DJ, Roth RL (2003) G-protein-coupled receptors at a glance. J Cell
Science 116: 4867-4869.
123. Agnati
LF, Ferre S, Leo G, Lluis G, Carde EI, et al. (2004) On the molecular basis of
the receptor mosaic hypothesis of the engram. Cell Mol Neurobiol 24: 501-516.
124. Triller
A, Choquet D (2005) Surface trafficking of receptors between synaptic and extra
synaptic membranes: “and yet they do move!” Trends Neurosci 28: 133-139.
125. Triller
A, Choquet D (2008) New concepts in synaptic biology derived from
single-molecule imaging. Neuron 59: 359-374.
126. Choquet
D, Triller A (2013) The dynamic synapse. Neuron 80: 691-703.
127. Lundstrom
K (2005) Structural genomics of GPCRs. Trends Biotechnol 23: 103-108.
128. Milligan
G, Canals M, Pediani JD, Ellis J, Lopez-Gimenez JF (2006) The role of GPCR
dimerisation/oligomerisation in receptor signalling. Ernst Schering Found Symp
Proc 2: 145-161.
129. Liu JD,
Kirti S, Luca Z, Chongguang C, Sean J, et al. (2018) In vivo brain GPCR
signalling elucidated by phosphoproteomics. Science 360: eaao4927.
130. Agnati LF, Baluska F, Barlow PW, Guidolin D
(2009) Mosaic, self-similarity logic, and biological attraction principles:
Three explanatory instruments in biology. Commun Integr Biol 2: 552-563.
131. Worth
CL, Kleinau G, Krause G (2009) Comparative sequence and structural analyses of
G-protein-coupled receptor crystal structures and implications for molecular
models. PloS One 4: e7011.
132. Vizi
ES, Fekete A, Karoly R, Mike A (2010) Non-synaptic receptors and transporters
involved in brain functions and targets of drug treatment. Br J Pharmacol 160:
785-809.
133. ViziES
(2013) Role of high-affinity receptors and membrane transporters in
non-synaptic communication and drug action in the central nervous system.
Pharmacol Rev 52: 63-89.
134. Yarnitzky
T, Levit A, Niv MY (2010) Homology modeling of G-protein-coupled receptors with
X-ray structures on the rise. Curr Opin Drug Discov Devel 13: 317-325.
135. Agnati
LF, Guidolin D, Albertin G, Trivello E, Ciruela F, et al. (2010) An integrated
view on the role of receptor mosaics at perisynaptic level: Focus on adenosine
A(2A), dopamine D (2), cannabinoid CB (1) and metabotropic glutamate mGlu(5)
receptors. J Recept Signal Transduct Res 30: 355-369.
136. Liebmann
C (2011) EGF receptor activation by GPCRs: A universal pathway reveals
different versions. Mol Cell Endocrinol 331: 222-231.
137. Kobilka
B (2013) The structural basis of G-protein-coupled receptor signaling (nobel
lecture). Angew Chem Int Ed Engl 52: 6380-6388.
138. Dulcis
D, Jamshidi P, Leutgeb S, Spitzer NC (2013) Neurotransmitter switching in the
adult brain regulates behavior. Science 340: 449-453.
139. Stevens
RC, Cherezov V, Katritch V, Abagyan R, Kuhn P, et al. (2013) The GPCR Network:
A large-scale collaboration to determine human GPCR structure and function. Nat
Rev Drug Discov 12: 25-34.
140. Simundza
J, Cowin P (2013) Adhesion G-protein-coupled receptors: Elusive hybrids come of
age. Cell Commun Adhes 20: 213-225.
141. Meshoulam
R, Parker LA (2013) The endocannabinoid system and the brain. Ann Rev Psychol
64: 21-47.
142. Chen L,
Duri KL, Gouaux E (2014) X-ray structures of AMPA receptor-cone snail toxin
complexes illuminate activation mechanism. Science 345: 1021-1026.
143. Fuxe K,
Tarakanov A, Romero Fernandez W, Ferraro L, Tanganelli S, et al. (2014)
Diversity and bias through receptor-receptor interactions in GPCR
heteroreceptor complexes. Focus on examples from Dopamine D2 receptor
heteromerization. Front Endocrinol (Lausanne) 5: 71.
144. Borroto-Escuela
DO, Brito I, Romero-Fernandez W, Di Palma M (2014) The G protein-coupled
receptor heterodimer network (GPCR-HetNet) and its hub components. Int J Mol
Sci 15: 8570-8590.
145. Sabbadin
D, Ciancetta A, Moro S (2014) Bridging molecular docking to membrane molecular
dynamics to investigate GPCR-ligand recognition: The human a2a adenosine
receptor as a key study. J Chem Inf Model 54: 169-183.
146. Alexander
SP, Davenport AP, Kelly E, Marrion N, Peters JA, et al. (2015) The concise
guide to pharmacology: G protein-coupled receptors. Br J Pharmacol 172:
5744-5869.
147. Dror
RO, Mildorf TJ, Hilger D, Manglik A, Borhani DW, et al. (2015) Structural basis
for nucleotide exchange in heterotrimeric G proteins. Science 348: 1361-1365.
148. Lee SM,
Booe JM, Pioszak AA (2015) Structural insights into ligand recognition and
selectivity for classes A, B and C GPCRs. Eur J Pharmacol 763: 196-205.
149. Hurevich
M, Talhami A, Shalev DE, Gilon C (2014) Allosteric inhibition of g-protein
coupled receptor oligomerization: Strategies and challenges for drug
development. Curr Top Med Chem 14: 1842-63.
150. Miller
AN, Long SB (2012) Crystal structure of the human two-pore domain potassium
channel K2P. Science 335: 432-436.
151. Brohawn
SG, del Mármol J, MacKinnon R (2012) Crystal structure of the human K2P TR+ AAK,
a lipid- and mechano-sensitive K+ ion channel. Science 335: 436-441.
152. Whorton
MR, MacKinnon R (2011) Crystal structure of the mammalian GIRK2 K+ channel and
gating regulation by G proteins, PIP2 and sodium. Cell 147: 199-208.
153. Jan LY,
Jan YN (1997) Cloned potassium channels from eukaryotes and prokaryotes. Annu
Rev Neurosci 20: 91-123.
154. Li W,
Aldrich RW (2011) Electrostatic influences of charged inner pore residues on
the conductance and gating of small conductance Ca2+ activated K+ channels.
Proc Natl Acad Sci USA 108: 5946-5953.
155. Wong
DT, Bymaster FP, Engleman EA (1995) Prozac (Fluoxetine, Lilly 110140), the
first selective serotonin uptake inhibitor and an antidepressant drug: Twenty
years since its first publication. Life Sci 57: 411-441.
156. Dong
YY, Pike AC, Mackenzie A, McClenaghan C, et al. (2015) K2P channel gating
mechanisms revealed by structures of TREK-2 and a complex with Prozac. Science
347: 1256-1259.
157. Inbeck
HG, Curioni A, Andreoni W (2000) Thiols and disulfides on the Au (111) surface:
The headgroup-gold interaction. J Am Chem Soc 122: 3839-3842.
158. Imoto
K, Busch C, Sakmann B, Mishina M, Konno T, et al. (1988) Rings of negatively
charged amino acids determine the acetylcholine receptor channel conductance.
Nature 335: 645-648.
159. Levitt
D (1991) General continuum theory for multi-ion channel. Biophys J 59: 271-277.
160. Neumann
E (2000) Digression on chemical electromagnetic field effects in membrane
signal transduction: Cooperativity paradigm of the acetylcholine receptor.
Bioelectrochemistry 52: 43-49.
161. Hasselmo
ME (2006) The role of acetylcholine in learning and memory. Curr Opin
Neurobiol16: 710-715.
162. Novere
NL, Corringer JP, Changeux JP (2002) The diversity of subunit composition in
nAChRs (acetylcholine receptors): Evolutionary origins. J Neurobiol 53:
447-456.
163. Changeux
JP (2012) Nicotinic acetylcholine receptor: The founding father. J Biol Chem
287: 40207-40215.
164. Youk,
H, Lim WA (2014) Secreting and sensing the same molecule allows cells to
achieve versatile social behaviors. Science 343: 1242782.
165. Auerbach A (2015) Agonist activation of a
nicotinic acetylcholine receptor. Neuropharmacology 96: 150-156.
166. Ho JMI,
Bennett MR (2018) Improved memory devices for synthetic cells. Science 360:
150-151.
167. Tadi
KK, Alshanski I, Mervinetskiy E, Marx G, Petrou P, et al. (2017)
Oxytocin-monolayer based impedimetric biosensor for zinc and copper ions. ACS
Omega 2: 8770-8778.
168. Alshanski
I, Blaszkiewicz J, Mervinetsky E, Rademann J, Yitzchaik S, et al. (2019)
Sulfation patterns of saccharides and heavy metal ion binding. Chem Eur J 25:
1-9.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- Advance Research on Alzheimers and Parkinsons Disease
- Journal of Pathology and Toxicology Research
- International Journal of Radiography Imaging & Radiation Therapy (ISSN:2642-0392)
- Advance Research on Endocrinology and Metabolism (ISSN: 2689-8209)
- Journal of Infectious Diseases and Research (ISSN: 2688-6537)
- International Journal of Medical and Clinical Imaging (ISSN:2573-1084)
- Journal of Allergy Research (ISSN:2642-326X)